An atom is 9% space. The whole mass of an atom is in the nucleus, and between nucleus and the edge of the atom, there are enormous distances. To understand it more if you increase the size of the nucleus to that of a coin the edge of the atom would be at a distance of 2-3 kilometer.
- Just how small are atoms? Really, really, really small. This fast-paced animation from TED-Ed uses metaphors (imagine a blueberry the size of a football stadium!) to give a visceral sense of just how small atoms are. Lesson by Jon Bergmann, animation by Cognitive Media.
- What does atom mean? The definition of an atom is the smallest component of an element, characterized by a sharing of the chemical properties.
- Student directions Build an Atom activity- homework version Learning Goals: Students will be able to 1. Make atom models that show stable atoms or ions. Use given information about subatomic particles to Identify an element and its position on the periodic table Draw models of atoms Determine if the model is for a neutral atom or an ion. Predict how addition or subtraction of a proton.
The key difference between cell and atom is that acell is made of molecules whereas atoms make up molecules.

Cells are the smallest functioning unit in a living organism. It contains many macromolecules. Meanwhile, atoms make up these macromolecules. Hence, an atom is the smallest unit of matter. Usually, a cell is on the micrometre scale while an atom is in the angstrom scale.
CONTENTS
1. Overview and Key Difference
2. What is a Cell
3. What is an Atom
4. Side by Side Comparison – Cell vs Atom in Tabular Form
5. Summary
An Atom Is
What is a Cell?
A cell is the smallest functioning unit of living organisms. In other words, it is the smallest unit of life. Therefore, we call it the building block of life. There are different types of cells such as microorganisms, animal cells and plant cells. They have different structures. But, all these cells have an outer membrane which holds the content inside.
When considering the structure of a cell, it contains a cytoplasm enclosed with a cell membrane, and the cytoplasm holds different organelles such as mitochondria. Therefore, different macromolecules, such as proteins, nucleic acid, carbohydrates, lipids, etc., make up the cells.

What is an Atom?
Atom is the smallest particle of a chemical element that can exist. Therefore, it is the smallest unit of matter, and a certain atom represents the properties of the chemical element to which it belongs. All gases, solid matter, liquids and plasma contain atoms. These are minute units; typically the size is around 100 picometers.
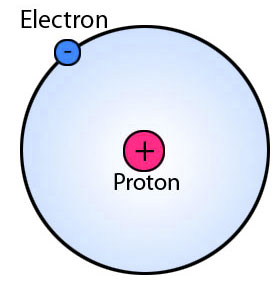
Figure 02: Typical Structure of an Atom
When considering the structure of an atom, it contains a nucleus and electrons moving around the nucleus. Further, protons and neutrons (and there are some other subatomic particles as well) make up the atomic nucleus. Typically, the number of neutrons, protons and electrons are equal to each other, but in the case of isotopes, the number of neutrons is different from that of protons. We call both protons and neutrons “nucleons”.
Around 99% of the atom’s mass is centred in the nucleus because the mass of an electron is almost negligible. Among these subatomic particles, a proton has +1 charge; an electron has -1 charge and a neutron has no charge. If the atom has equal numbers of protons and electrons, then the overall charge of the atom is zero; lack of one electron results in a +1 charge and a gain of one electron gives -1 charge to the atom.
What is the Difference Between Cell and Atom?
A cell is a biological unit, while an atom is a chemical unit. Besides, the key difference between cell and atom is that a cell is made of molecules whereas atoms make up molecules. Also, when considering the composition of these units, a typical cell contains cytoplasm, cell membrane, nucleus, etc. Sip migmate 100 manual. while an atom contains small subatomic particles such as electrons, protons and neutrons.
Moreover, a further difference between cell and atom is that a cell contains macromolecules such as proteins, carbohydrates, lipids and nucleic acid while molecules are made of atoms.
Summary – Cell vs Atom
A cell is a biological unit, while an atom is a chemical unit. In summary, the key difference between cell and atom is that a cell is made of molecules whereas atoms make up molecules.
An Atom Is Electrically Neutral
Reference:
1. Cooper, John A., et al. “Cell.” Encyclopædia Britannica, Encyclopædia Britannica, Inc., Available here.
Image Courtesy:
An Atom Is Composed
1. “Simple diagram of animal cell (en)” By domdomegg – Own work (CC BY 4.0) via Commons Wikimedia
2. “Atom Diagram” By AG Caesar – Own work (CC BY-SA 4.0) via Commons Wikimedia
Related posts:
What Is An Atom Quizlet
Is An Atom An Element
You probably already know everything is made upof little tiny things called atoms or even that each atomis made up of even smaller particles called protons, neutrons and electrons. And you've probably heardthat atoms are small. But I bet you haven't ever thoughtabout how small atoms really are. Well, the answer isthat they are really, really small. So you ask, just how small are atoms? To understand this,let's ask this question: How many atoms are in a grapefruit? Well, let's assume that the grapefruitis made up of only nitrogen atoms, which isn't at all true, but thereare nitrogen atoms in a grapefruit. To help you visualize this,let's blow up each of the atoms to the size of a blueberry. And then how bigwould the grapefruit have to be? It would have to be the same size of— well, actually, the Earth. That's crazy! You mean to say that if I filledthe Earth with blueberries, I would have the same numberof nitrogen atoms as a grapefruit? That's right! So how big is the atom? Well, it's really, really small! And you know what?It gets even more crazy. Let's now look inside of each atom— and thus the blueberry, right? — What do you see there? In the center of the atomis something called the nucleus, which contains protons and neutrons, and on the outside, you'd see electrons. So how big is the nucleus? If atoms are like blueberriesin the Earth, how big would the nucleus be? You might remember the old picturesof the atom from science class, where you saw this tiny dot on the pagewith an arrow pointing to the nucleus. Well, those pictures,they're not drawn to scale, so they're kind of wrong. So how big is the nucleus? So if you popped open the blueberryand were searching for the nucleus .. You know what? It would be invisible. It's too small to see! OK. Let's blow up the atom —the blueberry — to the size of a house. So imagine a ball that is as tallas a two-story house. Let's look for the nucleusin the center of the atom. And do you know what?It would just barely be visible. So to get our minds wrappedaround how big the nucleus is, we need to blow up the blueberry,up to the size of a football stadium. So imagine a ball the sizeof a football stadium, and right smack dabin the center of the atom, you would find the nucleus,and you could see it! And it would be the sizeof a small marble. And there's more, if I haven'tblown your mind by now. Let's consider the atom some more. It contains protons,neutrons and electrons. The protons and neutronslive inside of the nucleus, and contain almostall of the mass of the atom. Way on the edge are the electrons. So if an atom is like a ballthe size of a football stadium, with the nucleus in the center,and the electrons on the edge, what is in between the nucleusand the electrons? Surprisingly, the answer is empty space. (Wind noise) That's right. Empty! Between the nucleus and the electrons,there are vast regions of empty space. Now, technically there aresome electromagnetic fields, but in terms of stuff,matter, it is empty. Remember this vast region of empty spaceis inside the blueberry, which is inside the Earth, which really are the atomsin the grapefruit. OK, one more thing,if I can even get more bizarre. Since virtually all the massof an atom is in the nucleus — now, there is some amountof mass in the electrons, but most of it is in the nucleus — how dense is the nucleus? Well, the answer is crazy. The density of a typical nucleus is four times 10 to the 17thkilograms per meter cubed. But that's hard to visualize.OK, I'll put it in English units. 2.5 times 10 to the 16th poundsper cubic feet. OK, that's still kind of hard to figure. OK, here's what I want you to do. Make a box that is one footby one foot by one foot. Now let's go and graball of the nuclei from a typical car. Now, cars on average weigh two tons. How many cars' nuclei would youhave to put into the box to have your one-foot-box havethe same density of the nucleus? Is it one car? Two? How about 100? Nope, nope and nope. The answer is much bigger. It is 6.2 billion. That is almost equal to the numberof people in the Earth. So if everyone in the Earthowned their own car — and they don't — (Cars honking) and we put all of thosecars into your box .. That would be aboutthe density of a nucleus. So I'm saying that if you tookevery car in the world and put it into your one-foot box, you would have the density of one nucleus. OK, let's review. The atom is really, really, really small. Think atoms in a grapefruitlike blueberries in the Earth. The nucleus is crazy small. Now look inside the blueberry, and blow it up to the sizeof a football stadium, and now the nucleusis a marble in the middle. The atom is made upof vast regions of empty space. That's weird. The nucleus has a crazy-high density. Think of putting all those carsin your one-foot box. I think I'm tired.
